TRANSPORT INSIDE THE PERIVASCULAR BASEMENT MEMBRANE IN THE BRAIN
Alzheimer's disease is the seventh leading cause of death in the United States. The accumulation of beta-amyloid proteins in the vasculature walls of the brain is a characteristic of cerebral amyloid angiopathy and Alzheimer's disease. There is significant evidence suggesting that pathologies associated with beta-amyloid clearance failure in the brain contribute to the occurrence of Alzheimer's disease. However, the biomechanical mechanism of beta-amyloid clearance through the perivascular structures remains unclear. Studies reported in literature suggest that pulsating blood vessels may be associated with beta-amyloid clearance through this pathway. The arterial basement membrane is within the arterial wall that is separated with multiple layers of smooth muscles cells, and it is experimentally confirmed that the interstitial fluid carrying beta-amyloid in the arterial basement membrane is transported in the reverse direction of blood flow out of the brain. Furthermore, beta-amyloid clearance along the arterial basement membrane stops under cardiac arrest, suggesting that the driving force behind the reverse transport may be the peristaltic forward blood flow. A research group formed by myself, Dr. Paul Chiarot (Mechanical Engineering, Binghamton University), Dr. J. David Schaffer (Community and Public Affiars, Binghamton University), Dr. Roxana Carare (Cerebrovascular Anatomy, University of Southampton, U.K.), Dr. Nozomi Nishimura (Biomedical Engineering, Cornell University), and Dr. Chris Xu (Applied Physics, Cornell University), aims to study the mechanism that drives the reverse solute transport in the arterial basement membrane in healthy individuals and potentially explain how aging of the cerebral vasculature becomes a risk factor for Alzheimer's disease development. We have developed a preferential transport theory where reverse transport is hydrodynamically driven by the superposition of forward-propagating waves and their associated wave reflections along the arterial lumen. The forward-propagating waves are generated by the pulsation of the heart, while the reflection waves are created at the arterial branching junctions or any other sites with sudden changes in arterial geometry and/or elastic properties. This model helps predict the lack of interstitial fluid drainage following cardiac arrest in mice and incorporates physiological relevant parameters based on the extensive literature available for reflected boundary waves in blood vessels. The model also provides a means to gain insights into how the peri-arterial transport can be adversely affected by aging abnormalities such as basement membrane thickening and an increase in arterial wall stiffness.
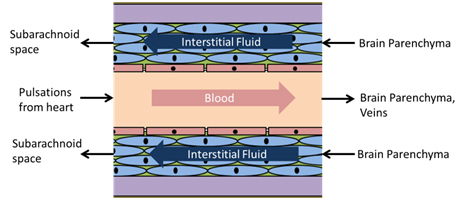 Fluid transport within a cerebral artery and the peri-arterial basement membrane |
DYNAMICS OF COATED DROPLETS INSIDE RESTRICTIVE GEOMERIES
Understanding and mitigating adverse environmental impact of hydraulic fracturing, a controversial but widely performed underground oil and gas extraction process, is of great technological importance to the environmentally responsible development and use of petroleum and natural gas resources. Surfactants, sand particles, and organic solvents are common additives to the large volume of fracking fluid pumped underground in this process; many of these additives have been suggested as major contaminants of drinking water. One of our ongoing research thrusts is investigating the dynamics of surfactant- or particle-coated droplets in restrictive geometries through integrated experiment, theory, and computational simulation. Our contribution to this field aims to provide an understanding of the adhesion of coated droplets to the rock pores which can lead to disastrous flow blockage and ensuing poor toxic chemical recovery. While bulk rheological properties of the surface-modified droplets and the dynamics of confined droplets transported by a continuous flow have been studied extensively, the underlying physical principles governing the initial stages of contact between static droplets and the confining walls remain poorly understood. Our objective is to favorably modify the interfacial boundary conditions on droplets and flow conduit walls such that control of the occurrence and the speed of their contact can be attained. We are currently using the level set and finite element methods to computationally model the multiphase fluid dynamics, ion transport, and electrostatic interactions between coated droplets and charged flow conduit walls. To complement computational modeling, droplet morphology and lubrication layer drainage will be monitored experimentally using evanescent wave imaging while the interface evolution equations are analytically derived and numerically solved. We are simultaneously collaborating with Dr. Xin Yong (Mechanical Engineering, Binghamton University), who is developing 3D mesoscale particle-based fluid simulations to capture the interplay between electrostatic interactions and interface-bound particle effect.
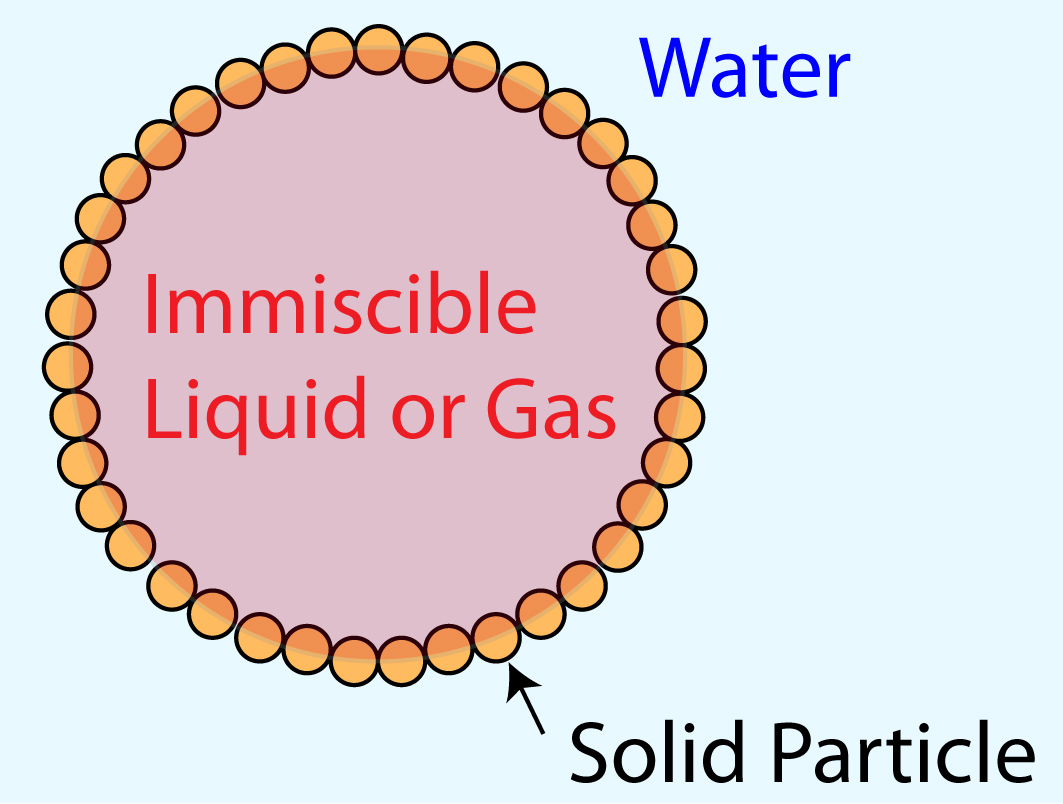 Armored droplet composition |
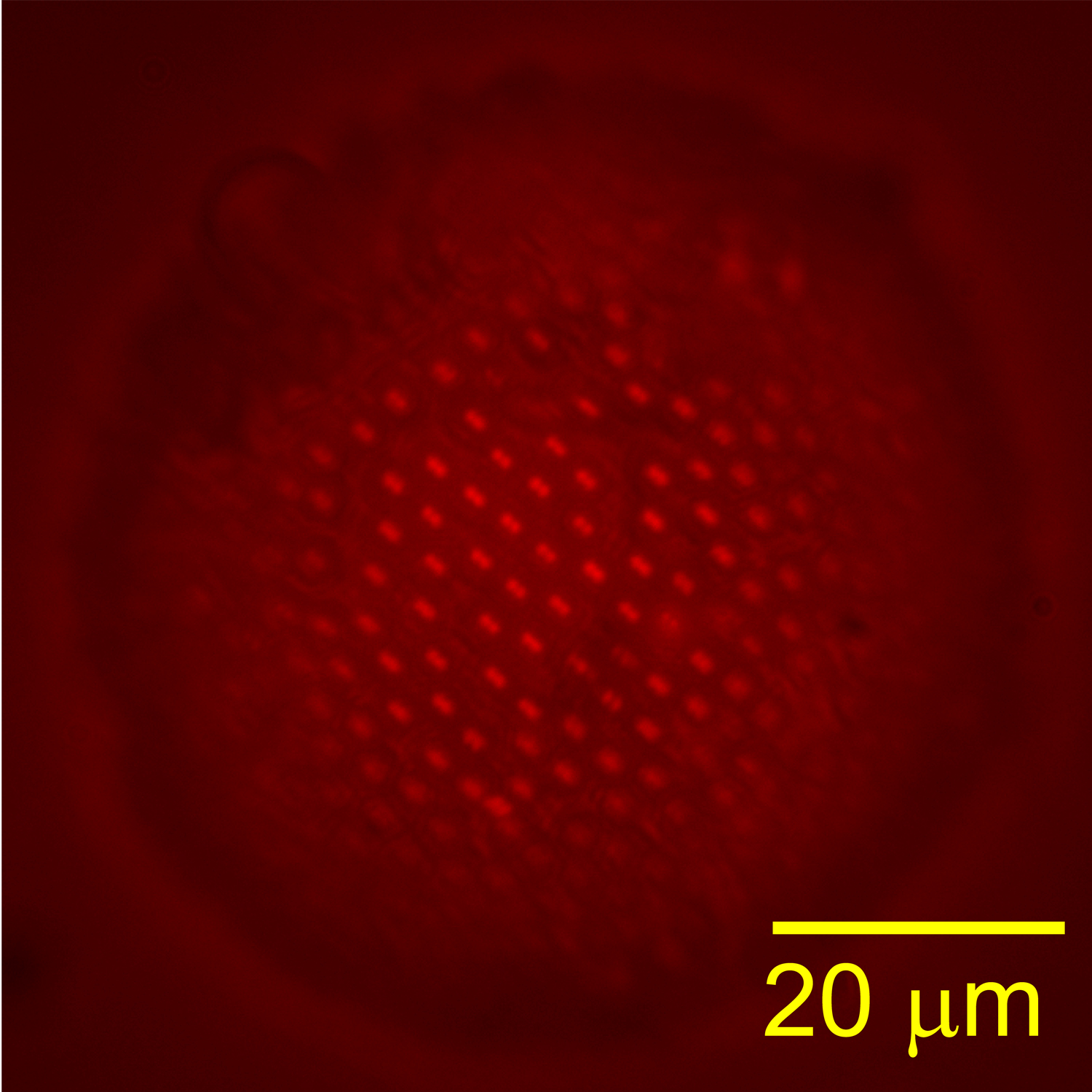 Fluorescent image of a Pickering emulsion droplet |
ENDOTHELIAL TO MESENCHYMAL TRANSFORMATION MECHANOBIOLOGY
The research objective of this work is to identify the role of mechanobiology on endothelial to mesenchymal transformation (EndMT) by examining aortic valve endothelial cell response to changes in the mechanical environment using static and microfluidic in vitro cultures and multiscale computational modeling. Endothelial cells line all blood-contacting surfaces of the circulatory system and are able to sense and respond to mechanical and biochemical signals in the blood, such as shear stress and inflammatory molecules, respectively. EndMT begins when a subset of endothelial cells delaminate from the cell monolayer, lose cell-cell contact and no longer exhibit endothelial markers. At the same time, these cells gain mesenchymal markers and develop an invasive and migratory tendency. The transformed cells that result from EndMT are involved in embryonic tissue development, in adult tissue homeostasis such as wound healing, and in adult pathologies including cancer metastasis, cardiac fibrosis, and calcific aortic valve disease. In collaboration with Dr. Gretchen Mahler (Biomedical Engineering, Binghamton University) and Dr. Bruce Murray (Mechanical Engineering, Binghamton University), we are studying the role of mechanobiology (specifically extracellular matrix composition, shear stress, and inflammation) on EndMT. In this work, we fabricated a microfluidic device that is specifically designed to carry out in vitro experiments to determine the mechanism by which altered 3D extracellular matrix composition and mechanical properties, altered shear stress, and/or inflammatory signaling drives EndMT. Simultaneoulsy, we are developing a hybrid continuum-stochastic cell level model capable of simulating multiple aspects of mesenchymally transformed cell evolution based on the nature of the surrounding chemical and mechanical environment. In this stochastic model, EndMT and invasion are initiated when cell-to-ECM and cell-to-cell adhesion forces fall under pre-defined threshold values. Trajectories of cell migrations are computed based on biased random walks where directions and magnitudes of cell invasion are influenced by their affinity to nutrients and avoidance of waste molecules and ECM resistance. Integrated computational programs of molecular diffusion (finite-difference method), EndMT initiation (stochastic), and cell migration (biased random walks) have been developed and are currently being tested for validity with in vitro experiments conducted in the microfluidic devices.
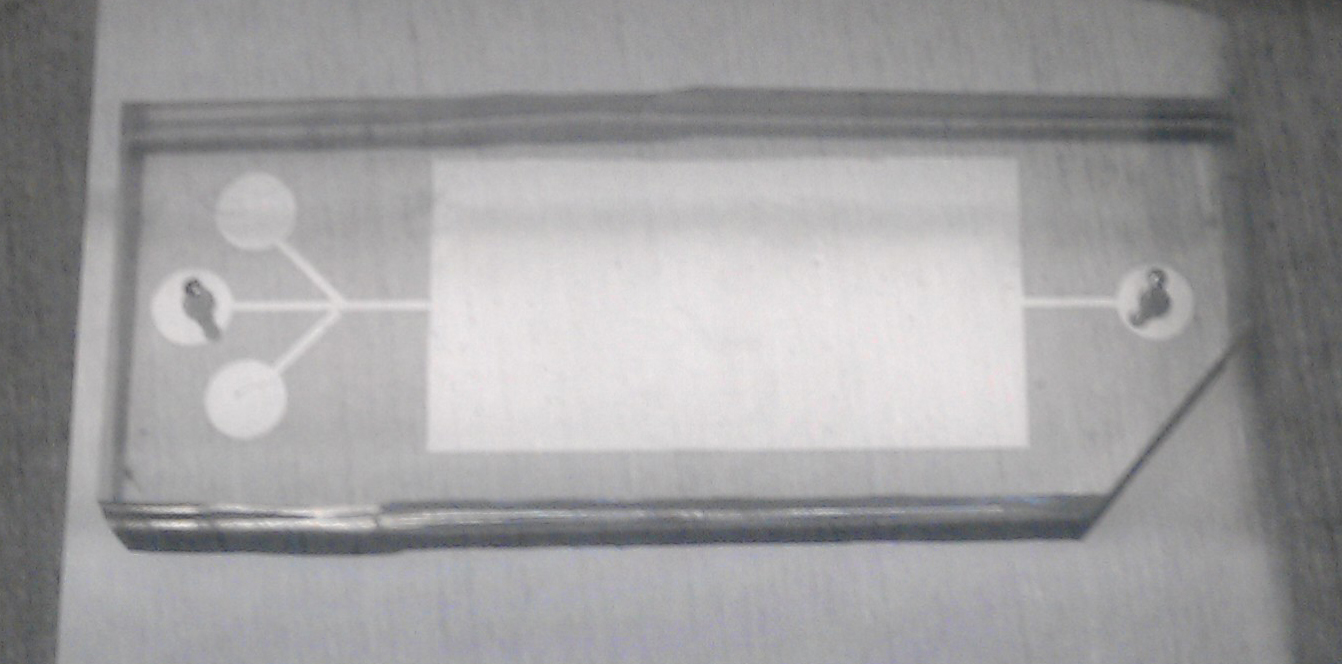 A microfluidic chamber for 3D-ECM incubation and experiment |
 Human umbilical vein endothelial cells in the microfluidic chamber |
EPIGENETIC REGULATION OF SURFACTANT PROTEINS IN LUNG CANCER
Lung cancer is the most common form of noncutaneous malignancy and has the highest cumulative cancer mortality each year, accounting for 18% of total cancer deaths. The human lung is constantly exposed to various environmental factors including pathogens and air pollutants, which have been linked to lung cancer. Air pollutants and various carcinogens can induce DNA changes such as DNA methylation. There is a thin layer of lipids and surfactant protein complex that line the fluid-air interface of the alveoli and protect the lung from environmental insults. Surfactant proteins are expressed in the lung and play an important role in host defense and the regulation of pulmonary inflammatory processes. Thus, DNA alteration of surfactant protein genes is a key event in the pathogenesis of lung diseases including lung cancer. In collaboration with Dr. Guirong Wang (Cancer Research Institute, SUNY Upstate Medical University) and Dr. Gretchen Mahler (Biomedical Engineering, Binghamton University), we are designing a miniature model of the lung to explore the effect of DNA alternation and surfactant protein expression in human epithelial cell lines following exposure to physiologically realistic doses of air pollution particulate matter. The gained knowledge will help better understand the pathogenesis of lung cancer, and may provide novel targets/biomarkers for the diagnosis and therapy of lung cancer.
OPTICAL DIAGNOSTIC TECHNIQUES FOR MICRO/NANOFLUIDICS
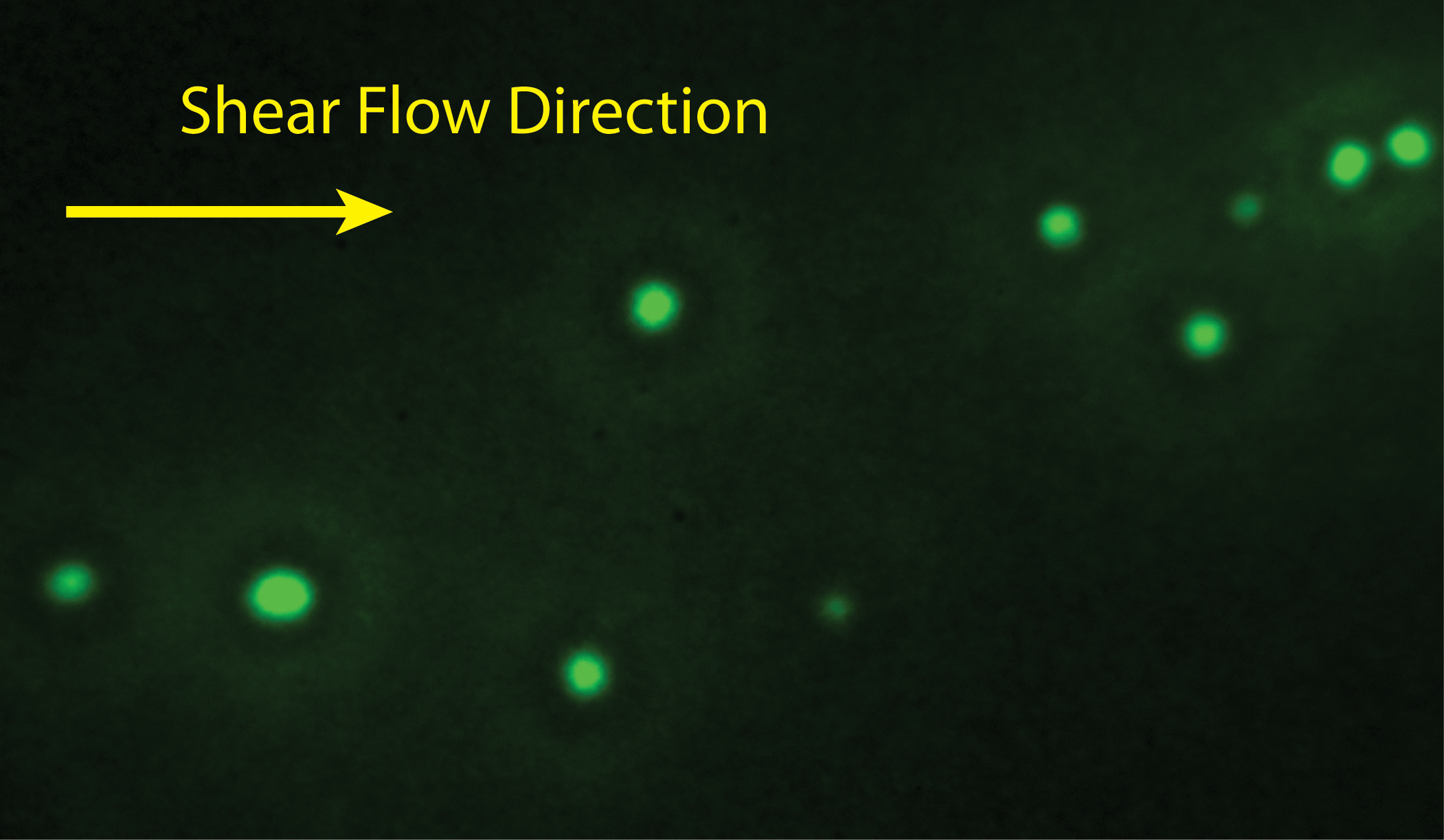
Tracer particle-based image velocimetry is a widely used technique to visualize flow field in the macroscopic scale. Optical microscopy and microscale particle image velocimetry (µPIV) are insufficient for nanoscale investigations due to large optical resolutions (~0.5 µm) and their limit on two-dimensional imaging with restricted depth-resolving power. Recently Dr. Huang developed a novel evanescent wave-based imaging technique termed three-dimensional total internal reflection velocimetry (3D-TIRV) for conducting three-dimensional fluidic and colloidal measurements with a 10-nm displacement resolution. In 3D-TIRV, fluorescent tracer particles within 1 µm from a two-medium interface are illuminated with an evanescent field. Under this configuration, the emission intensities of the fluorescent particles decay exponentially as a function of distance to the interface. Thus the in-plane displacement of a tracer particle can be tracked with standard particle tracking velocimetry while its out-of-image-plane motion can be tracked with its intensity. In our recent work, we advanced the technique with a series of numerical and experimental investigations into identifying measurement bias and improving the accuracy of the evanescent wave particle velocimetry technique. We identified the shear-induced and depletion layer-related bias and developed an enhanced intensity analysis algorithm to reduce the effects due to intrinsic tracer size variations.
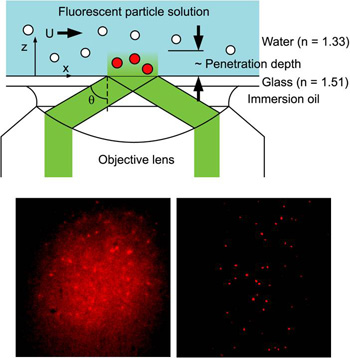 Top: Schematic of 3D-TIRV. Bottom: Flood (left) and evanescent wave (right) illumination of 200-nm Fluorescent Particles |
 Our 3D-TIRV system built on a Nikon TE-2000U microscope |
Last Updated: 3/4/16