|
High-throughput Sensing Arrays
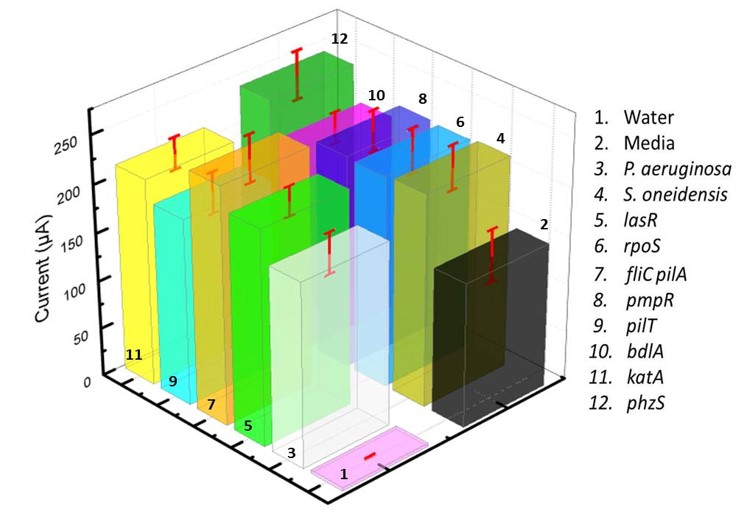
|
There
is a large global effort to improve microbial fuel cell (MFC) techniques and
advance their translational potential toward practical, real-world
applications. Significant
performance breakthroughs cannot be achieved through naturally existing species
without the power of genetic engineering techniques, either maximizing a
specific organismí»s electrogenic potential, stitching together genes involved
in electrogenesis from various organisms or a bacterial electrogenic consortium. Although substantial research
has been conducted on genetic engineering of microbial metabolic pathways for
biofuel generation, the genetic approaches for their higher electricity generation is quite
limited to date. This is mainly due to the limitations in current screening
methods for the bacterial electrical properties, while microbial
biofuel-producing capacity can be readily performed by using well-established
microarray techniques, which are widely used to monitor gene expression under different cell
growth conditions and detect specific mutations in DNA sequences. Microbial screening arrays for
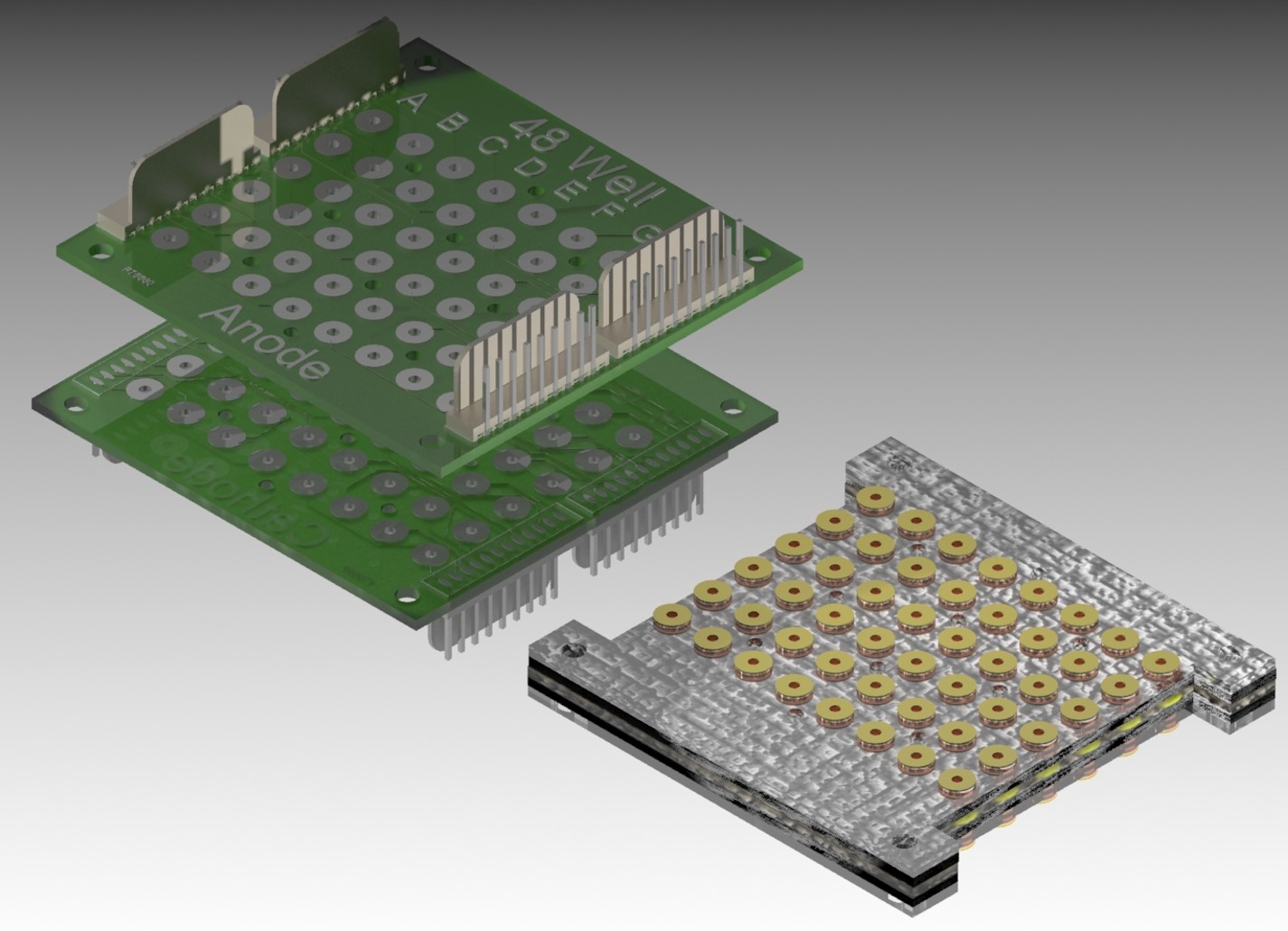
bioelectricity generation require much more complicated device configurations and
fabrications, which include an active feeding system and dual chambers
separated by a proton exchange membrane, compared to the general microbial microarray
including only one chamber without any electrical measurements. Recently developed MFC arrays
have complex MFC architectures with many tubings/channels that operate with
external pumps, constraining the number of distinct wells on the array only to
24. If a
24-well MFC array was designed to have a two-chambered configuration requiring
individual anolyte/catholyte inlets and outlets, 96 tubing ports and fluidic
pathways would have to be implemented and operated by several multichannel
syringe or peristaltic pumps. The electrical contacts for electrical
characterization of the MFC units may increase the complexity of the device
architecture. Furthermore, each MFC unit requires long start-up times for
bacterial accumulation and acclimation as biofilms adhere to the anode. These limitations have motivated us
to develop a new conceptual MFC array, such that the high-throughput and rapid
power assessment can be significantly improved with a compact and simple device
design. Currently, we are developing a paper-based
microbial sensor array as a high-throughput, rapid screening tool for microbial
electricity generation study.
|

